There is nothing worse than handing out a diagnosis for a debilitating rare disease which has no treatment. At RxMD we find it gratifying to work on rare diseases and have worked/are working on Huntington disease, Gorlin syndrome, recessive dystrophic epidermolysis bullosa, sarcoidosis and Netherton syndrome.
Rare diseases are defined as conditions with a prevalence of ≤ 1/1000. Rare diseases can be varied, however, our interest is in rare monogenetic diseases; and while there are several therapeutic options for rare monogenetic diseases, this blog is about RNA therapeutics.
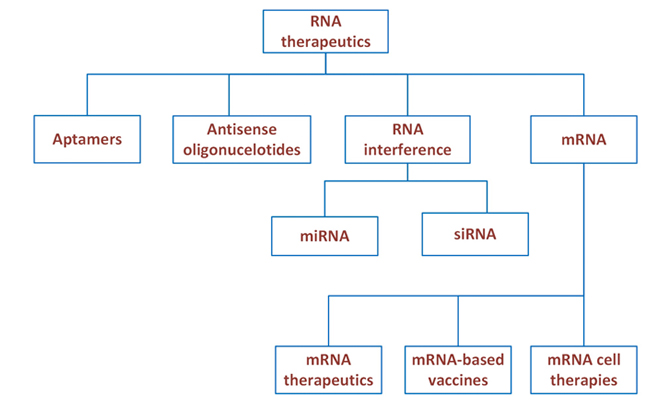
RNA therapeutics comprise varied nucleic acid-based strategies such as RNA aptamers, antisense oligonucleotides (ASO), RNA Interference (RNAi) and messenger RNA (mRNA)-based therapeutics. RNAi could be short interfering RNA (siRNA) or micro-RNA (miRNA). The site of action for ASO and miRNA-based therapeutics is the nucleus, for siRNA inhibitors and mRNA-based therapeutics, it is the cytoplasm; aptamers can bind targets in any of these areas. While aptamers can be delivered directly, other RNA therapeutics are combined with aptamers or antibody fragments, or coated with nanoparticles with receptor-specific ligands, to allow for the targeted delivery to the site of action.
Aptamers may come from diverse nucleic acid pools, directly bind extracellular, cell surface, or intracellular proteins with high specificity and affinity. They can be likened to agonists. They are not complementary in base sequence to an RNA strand but are useful due to their tertiary structure. Pegaptanib an aptamer, is a VEGF inhibitor used in neovascular age-related macular degeneration.
ASO’s are modified DNA of up to 30 nucleotides. They prevent mRNA translation by blocking the start of translation or tagging the mRNA for degradation, e.g., inotersen for TTR amyloidosis. They can also splice the pre-mRNA strand to exclude mutant genes (exon skipping) e.g. eteplirsen for Duchenne muscular dystrophy.
RNAi could be siRNA or miRNA. siRNA are usually double stranded, 21-base pair long, made up of the drug strand (anti sense) and a delivery strand (sense strand). Patisiran the first approved siRNA is indicated for the treatment of hereditary transthyretin amyloidosis.
miRNAs are small non-coding RNA molecules that regulate the expression of multiple mRNAs by blocking translation or promoting degradation of their target mRNAs. There are currently no approved drugs using this technology.
mRNA based therapeutics comprise mRNA that encode certain peptides or proteins which elicit their expression in the cytoplasm (e.g., to replace defective proteins or present antigens for vaccination). They can be used for protein replacement therapy or vaccination.
The routes of administration for these therapeutics vary. In primary hyperoxaluria, the organ targeted is the liver. Lumasiran is an siRNA given subcutaneously with an N-acetyl-D-galactosamine ligand directed to liver cells, the enzyme of the alternate pathway is deactivated, and formation of oxalate prevented. In spinal muscular atrophy, the SMN protein, though expressed by most cells, is primarily present in anterior horn cells. Nusinersen is therefore given intrathecally. In neuronal ceroid lipofuscinosis type 2 there is accumulation of peptides due to absence of a serine protease enzyme. Though present in organs other than brain and retina, its main expression and, hence, accumulation is in brain. Delivery across the blood brain barrier (BBB) is a challenge and the developing RNA therapeutic may need to be given intraventricularly/intracerebrally.
Overall, this is an evolving field and holds out a lot of promise for rare monogenetic conditions.
For more blogs, please visit: https://www.rxmd.com/insights