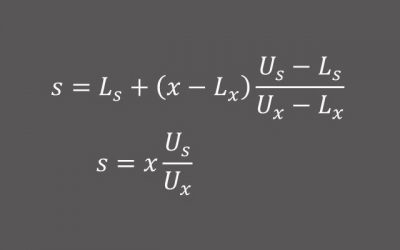What We Do
Our skills span translational medicine, clinical development and drug safety – for small molecules, biologics and cell therapy. Our expertise encompasses strategy and execution.

Translational Medicine
Our physicians identify and bridge the gaps from in vitro pharmacology to commercialization.
Clinical Development
At every step, in every stage of clinical drug development – be it formulating the clinical development plan or first in Human, Phase 2 or pivotal Phase 3 trials – we have a skill that can be unflinchingly relied upon.
Drug Safety
We provide a wide spectrum of safety science (not operations) services in both pre-approval and post-approval stages to ensure there is no compromise on signal detection.
Client Speak

Usman "Oz" Azam, MD
In the cell and gene therapies world, RxMD have developed a very credible scientific, translational and development understanding. At Novartis during 2013-2016, my teams

Joseph H. Hoffman, MD
It is a pleasure to commend RxMD to any pharmaceutical company, large or small, that is in need of additional medical support in pursuit of its clinical research objectives.
Insights
Lab Data Normalization
In a recent development program, the Sponsor was stumped when the central laboratory for the pivotal registration study shut down its services midway through the study. The Sponsor quickly identified another central...
Translational Medicine: Mind the Gap
We are constantly reviewing documents targeting the FDA and EMA. On reviewing a recent pre-IND briefing book for the U.S. FDA, we were struck by some translational gaps in the story board. While these were not significant,...
Milestones
Our Clientele
We have worked for U.S. and EU-based big pharma & biotech, for small molecules, biologics & cell therapy, across indications.