This blog is largely excerpted from an excellent guidance from the FDA: Non-Inferiority Clinical Trials to Establish Effectiveness – Guidance for Industry. Here we look at aspects of the guidance relevant to the clinical scientist.
FDA’s regulations describe four kinds of concurrently controlled trials that provide evidence of effectiveness. Three of them — placebo, no treatment, and dose-response controlled trials — are Superiority Trials. When these three trial designs are not feasible or considered unethical, the fourth kind, comparison with an active control, may be designed to demonstrate effectiveness. This may be a Superiority Trial or a Non-Inferiority (NI) Trial.
We use three terms, T for the test drug whose effectiveness is sought to be demonstrated, C for the active control whose effectiveness has been demonstrated, and P for placebo.
NI trials were once called clinical equivalence trials. The intent of an NI trial, though, is not to show equivalence. It is intended to show that a new treatment T that demonstrates non-inferiority to C is effective (versus P), not that it is as effective as C. In an NI trial, no conclusion can be drawn between T and C unless it is pre-specified that the trial will also evaluate superiority between T and C.
NI is established by showing that T has an effect sufficiently close to the effect of C. There is no P in the NI study; therefore, the effect of C (versus P) is not measured in the study but must be assumed. The goal of the study is to show that the effect of T is not inferior to C by a specified amount, called the NI margin, or M.
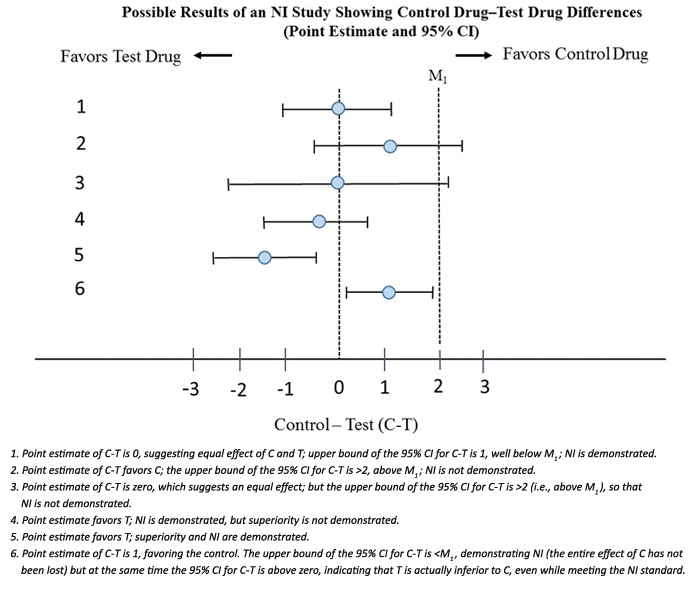
M can be no larger than the presumed entire effect of C, i.e. M cannot be < than the point estimate for C-P based on past randomized placebo controlled trials for C versus P. When M is set equal to the point estimate of C-P assumed to be present in the NI study, it is referred to as M1. Showing that the upper bound of the 95% CI of C-T is less than M1 demonstrates that the test drug has some effect (i.e., an effect > 0).
In order for an NI trial to be interpretable, the NI trial design must be similar to the study comparing C versus P. The current effect of the active control in the NI study must be similar to that observed in past studies. If, for example, the NI margin is chosen as 10, and the study does indeed rule out a difference of 10 (but not a smaller difference), seeming to demonstrate effectiveness of T, but the true effect of C in this study was actually less than 10, then a conclusion that the study demonstrated non-inferiority would have been incorrect.
The figure from the FDA guidance is self-explanatory. M1 = 2 is the point estimate for C-P in the placebo versus control trial. The figure puts things into perspective from both an NI and superiority perspective.
The margin of interest, however, is usually smaller than M1 (to show that an adequate portion of the clinical benefit of the control is preserved), in which case it is called M2. But that is a topic for another blog.
Reference: U.S. Food and Drug Administration (FDA). Non-Inferiority Clinical Trials to Establish Effectiveness – Guidance for Industry: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/non-inferiority-clinical-trials
For more blogs, please visit: https://www.rxmd.com/insights