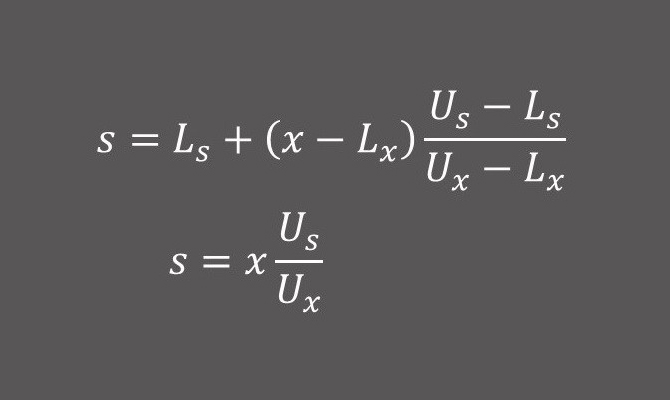In a recent development program, the Sponsor was stumped when the central laboratory for the pivotal registration study shut down its services midway through the study. The Sponsor quickly identified another central laboratory.
The Sponsor ended up with most subjects having their initial lab results from lab A, with subsequent lab tests for the same parameters from lab B for a different lab normal range. Now, most regulators expect to see laboratory data summarized in three ways: measures of central tendencies, shifts, and extreme outliers.
A change in the laboratory midway through the program precluded reporting of measures of central tendencies, as summarizing lab values with different lab normals defeated the purpose, for it introduced a systemic bias with baseline labs from lab A and end of treatment labs from lab B. We recommended a technique we had used several times in the past for regulatory submissions.
In a pediatric development program encompassing a wide age range, we had age specific normal ranges for laboratory parameters. In another development program, we used local laboratories, again with laboratory specific lab normal ranges. In both situations, we used transformation using the methodology outlined in Karvanen et al (2003: DOI:10.1177/009286150303700112) to normalize the lab value to the new reference range.
If the location-scale family transformation (Eq. 1) resulted in a negative number, we used the scale family transformation (Eq. 2) instead.
Let assay values x and s have reference ranges (Lx, Ux) and (Ls, Us)
For more blogs, please visit: https://www.rxmd.com/insights