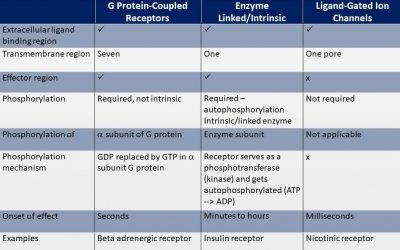
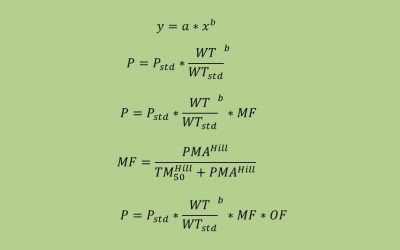Cell Surface Receptors
Receptors can be classified in various ways. Based on their function they can be classified as either “cargo” type receptors or signal (transduction) receptors. Cargo-type receptors deliver key metabolic substrates,...
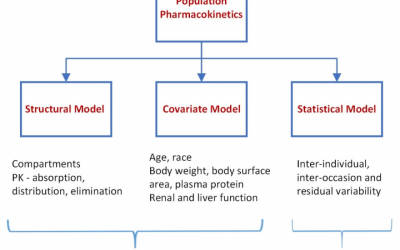
Population PK for the Physician Scientist
In previous blogs we highlighted the utility of an exposure response (E-R) analysis to demonstrate efficacy. In an E-R analysis the relationship between the amount of drug exposure (typically area under concentration-time...
Pediatric Drug Development: Four Critical Considerations
Drug development in children can be daunting. There are four key considerations: Safety, Pharmacokinetics (PK), Pharmacodynamics (PD) and the indication. Safety: In adults, adverse events (AEs) may be related to...
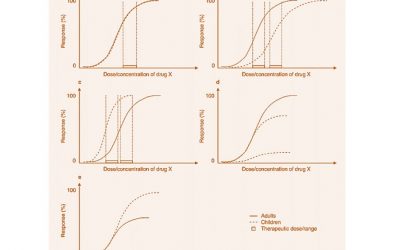
Pediatric Drug Development: Developmental Pharmacodynamics
Developmental Pharmacodynamics (PD) constitutes the study of age related maturation of the structure and function of various organ systems and how this impacts pharmacologic response....
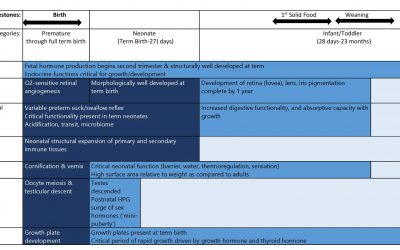
Pediatric Drug Development: Safety Concerns – Initial Two Years
This blog is a continuation of a previous blog on the safety issues that might be uncovered in the pediatric population. Here we look at susceptibilities particular to the first two years of life for some key organ...
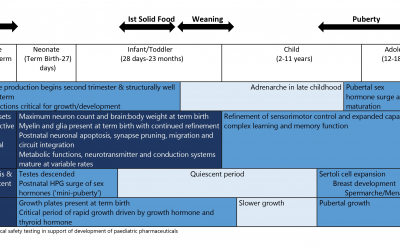
Pediatric Drug Development: Safety Considerations Beyond Age Two
This blog evaluates the potential for unanticipated Adverse Events (AEs) in children >2 years of age. The one aspect of pediatric drug development which is a major concern is drug safety. While children, at exposures...
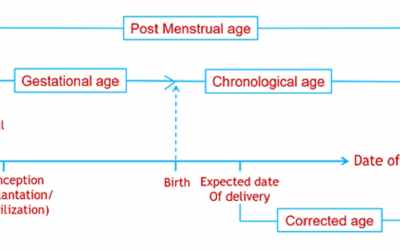
Pediatric Drug Development: PK
This blog, adapted largely from an excellent article on the subject by Anderson et al., targets the pediatrician scientist who is wont to think that children are not small adults. As will be seen here and in the original...
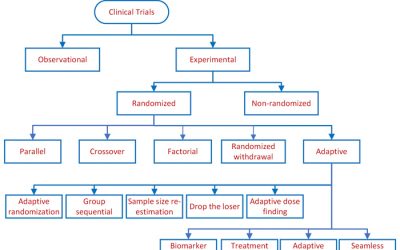
Clinical Study Designs
Clinical study designs are of two major types: observational and experimental, as depicted above. Observational studies seek to generate hypotheses, while experimental studies seek to test hypotheses. Experimental studies...
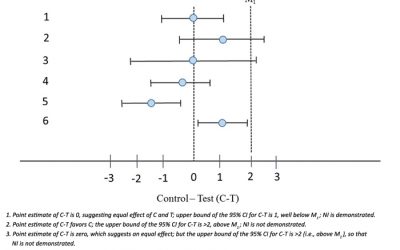
Non-Inferiority Trials – Clinical Development Perspective
This blog is largely excerpted from an excellent guidance from the FDA: Non-Inferiority Clinical Trials to Establish Effectiveness - Guidance for Industry. Here we look at aspects of the guidance relevant to the clinical...
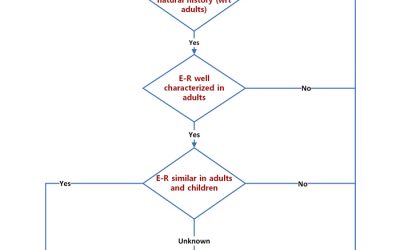
Pediatric Drug Development: Optimizing Extrapolation
Optimizing the use of the extrapolation concept in pediatric investigational programs: Here are 4 key considerations. Drug development in pediatrics continues to be a substrate for the application of innovative tools and...
Our Clientele
We have worked for U.S. and EU-based big pharma & biotech, for small molecules, biologics & cell therapy, across indications.