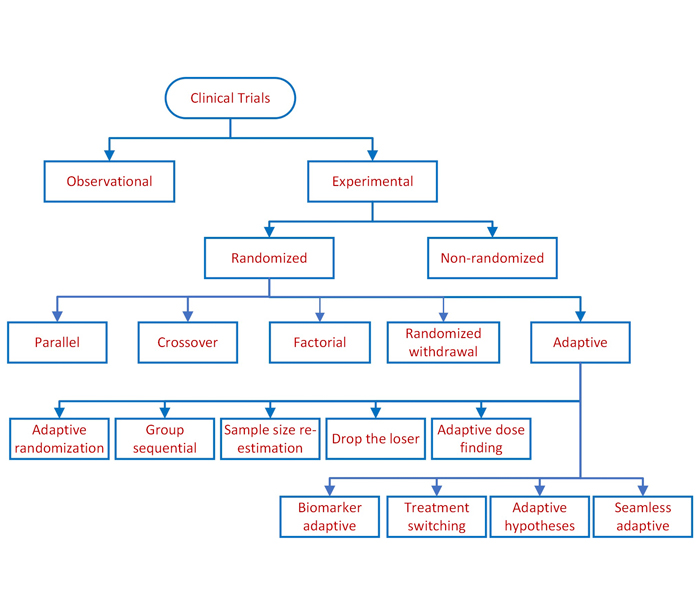
Clinical study designs are of two major types: observational and experimental, as depicted above. Observational studies seek to generate hypotheses, while experimental studies seek to test hypotheses. Experimental studies involve studying the association between exposure to an intervention and the outcome from such exposure.
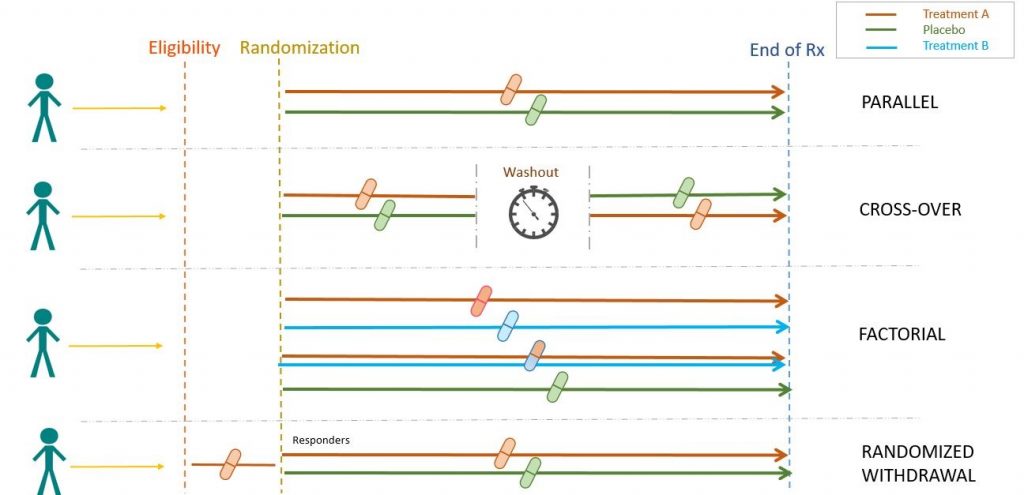
Experimental studies are further divided into non-randomized and randomized clinical trials. Non-randomized clinical trials involve selecting controls without randomization. Randomized clinical trials, however, involve randomizing subjects, as presented below.
An adaptive design allows modifications to the trial and/or statistical procedures of the trial after its initiation, without undermining its validity and integrity. The purpose is to make clinical trials more flexible, efficient and fast. Based on adaptations employed, they can be classified by design methods or by categorizing these methods under different rules.
An adaptive randomization design involves alterations made in the randomization schedule, depending upon the varied or unequal probabilities of treatment assignment (e.g. DexFEM trial – low dose dexamethasone for women with heavy menstrual bleeding).
A group sequential design not only facilitates cessation of a trial prematurely if safety or efficacy issues are detected but also, further modifications, depending upon the results of the interim analysis. An example is the “3+3” Phase I trial design for finding the maximum-tolerated-dose (e.g. PARADIGM-HF trial– sacubitril + valsartan against enalapril for heart failure).
A sample size re-estimation design allows modification or re-estimation of the sample size, determined by the interim observed data. Such adjustment may be done blinded or unblinded, based on the criteria of treatment effect-size, conditional power and/or reproducibility probability (e.g. SPS3 study examining the relationship between anti-platelet therapy and recurrent stroke, and between systolic blood pressure control and recurrent stroke in a 2+2 factorial design).
In drop-the-loser design, the dose arms detected to have received inferior treatments at the interim analysis can be dropped out (e.g. ACTIW trial evaluating therapies in combination or sequentially with tyrosine kinase inhibitors in chronic myelogenous leukemia patients).
An adaptive dose-finding design is often used in early-phase clinical development to identify the minimum effective dose and the maximum tolerable dose, which is used to determine the dose level for the next phase clinical trial (e.g. DILT1D study of IL-2 dose on regulatory T cells in type 1 diabetes).
In a biomarker-adaptive design modifications may be made in the ongoing trial, based on the response of various biomarkers associated with the disease under consideration. In a treatment-switching design, shifting the patient from one treatment option to other is allowed if there are safety or efficacyconcerns (e.g. BREAK-3 trial evaluating dabrafenib versus dacarbazine in untreated BRAF V600E mutation-positive melanoma patients).
An adaptive-hypotheses design facilitates modifications or changes in hypotheses based on interim analysis results. In a seamless-adaptive design, a Phase II trial transition into a Phase III trial happens without pause, saving considerable drug development time (e.g. GBM AGILE trial evaluating multiple regimens for newly diagnosed and recurrent glioblastoma)
References:
Mahajan R, Gupta K. Adaptive design clinical trials: Methodology, challenges and prospect. Indian J Pharmacol. 2010 Aug;42(4):201-7.
Food and Drug Administration (FDA). Guidance for Industry. Adaptive Design Clinical Trials for Drugs and Biologics. Dec 2019. Available from: https://www.fda.gov/media/78495/download
For more blogs, please visit: https://www.rxmd.com/insights