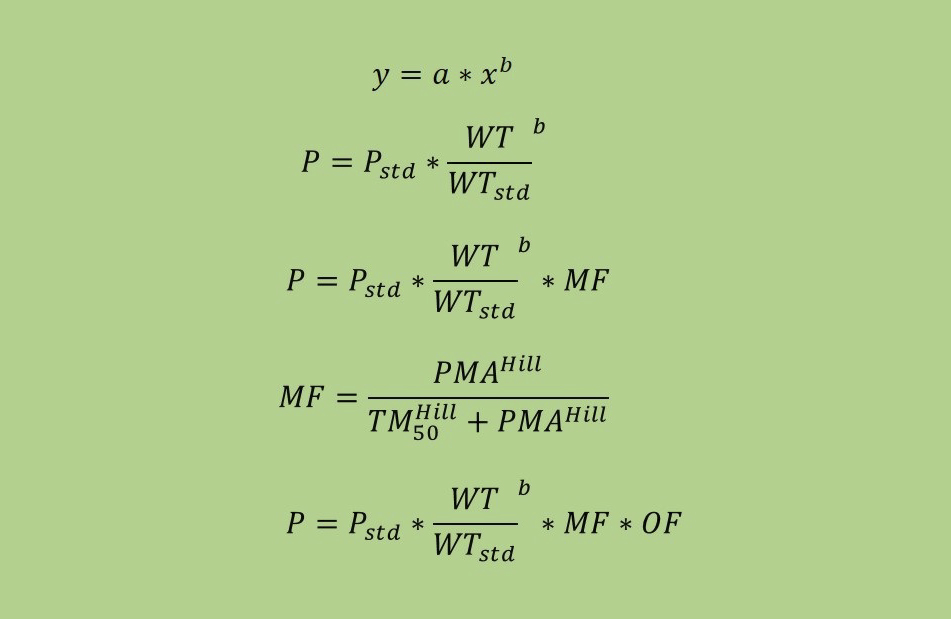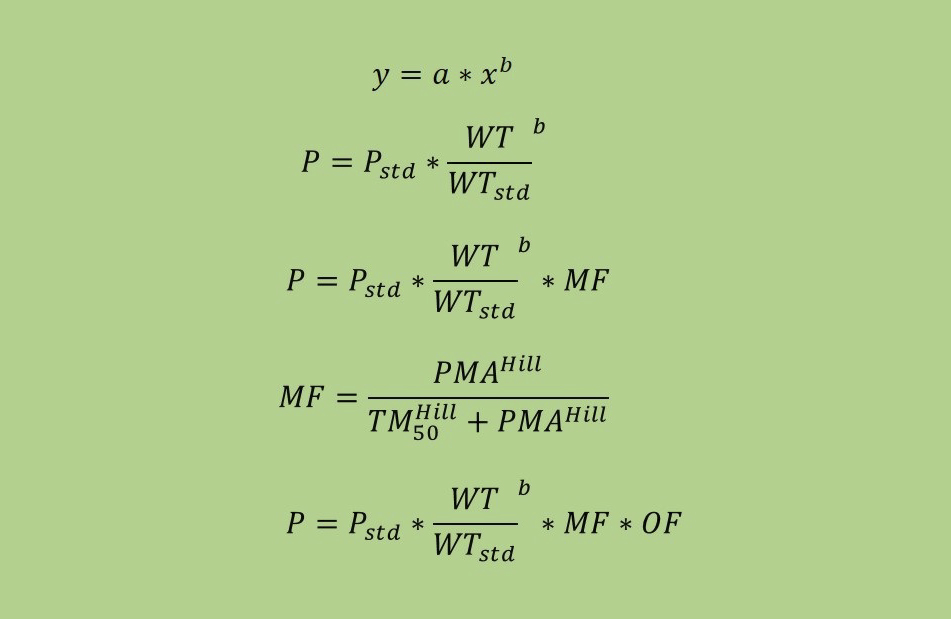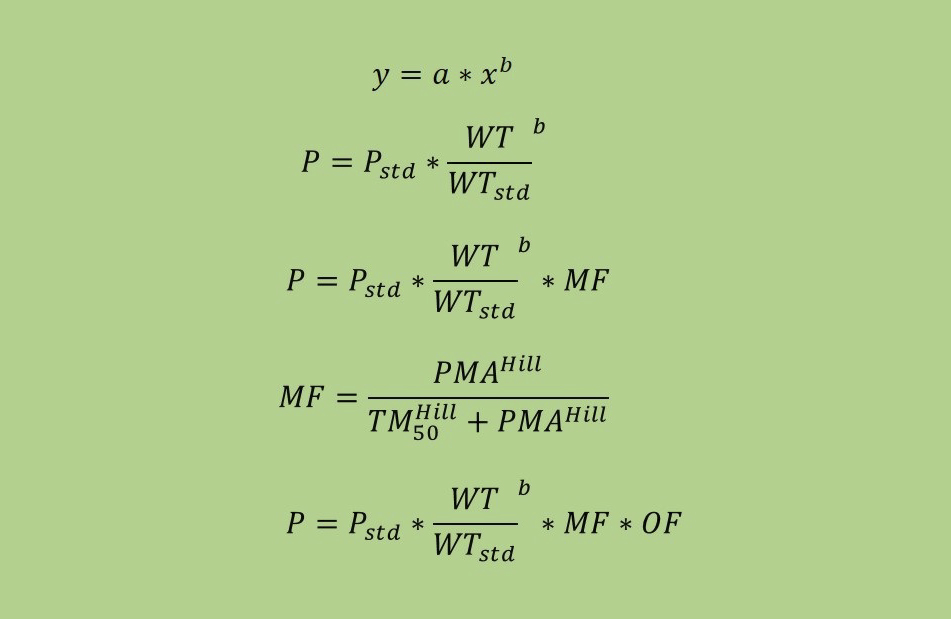
by admin | May 2022 | Uncategorized
This blog, adapted largely from an excellent article on the subject by Anderson et al., targets the pediatrician scientist who is wont to think that children are not small adults. As will be seen here and in the original article, there is lot to pediatric...
by admin | March 2022 | Uncategorized
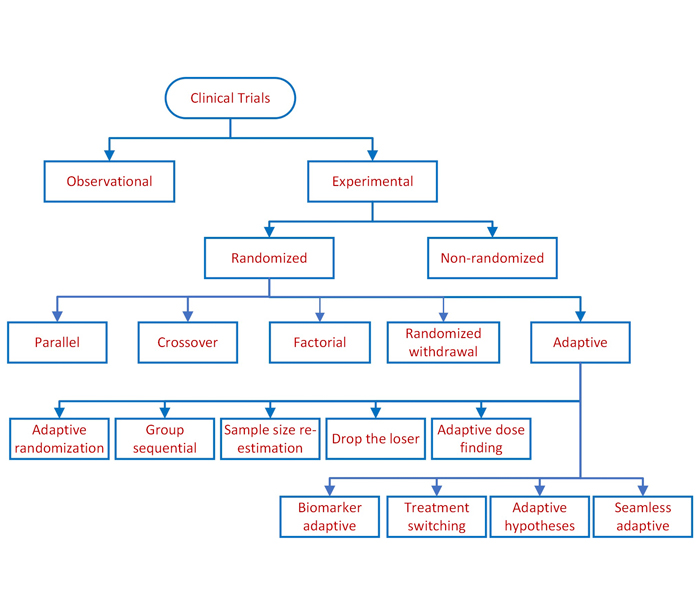
Clinical study designs are of two major types: observational and experimental, as depicted above. Observational studies seek to generate hypotheses, while experimental studies seek to test hypotheses. Experimental studies involve studying the association between...
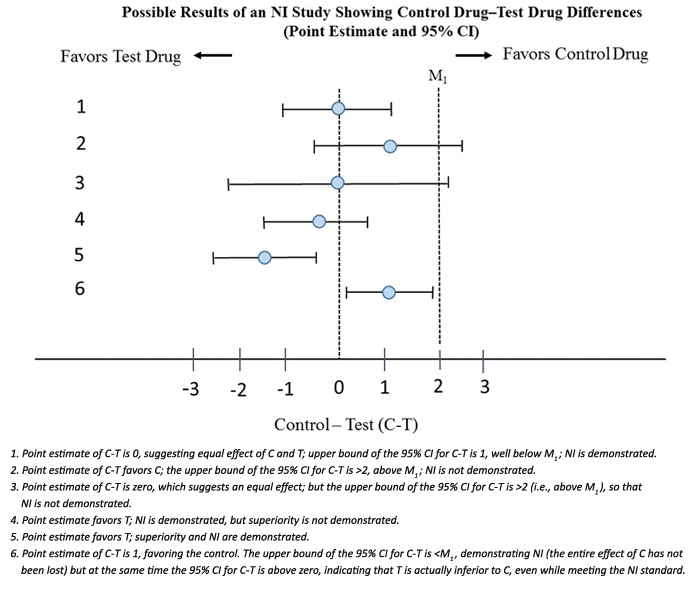
by admin | March 2022 | Uncategorized
This blog is largely excerpted from an excellent guidance from the FDA: Non-Inferiority Clinical Trials to Establish Effectiveness – Guidance for Industry. Here we look at aspects of the guidance relevant to the clinical scientist. FDA’s regulations describe...
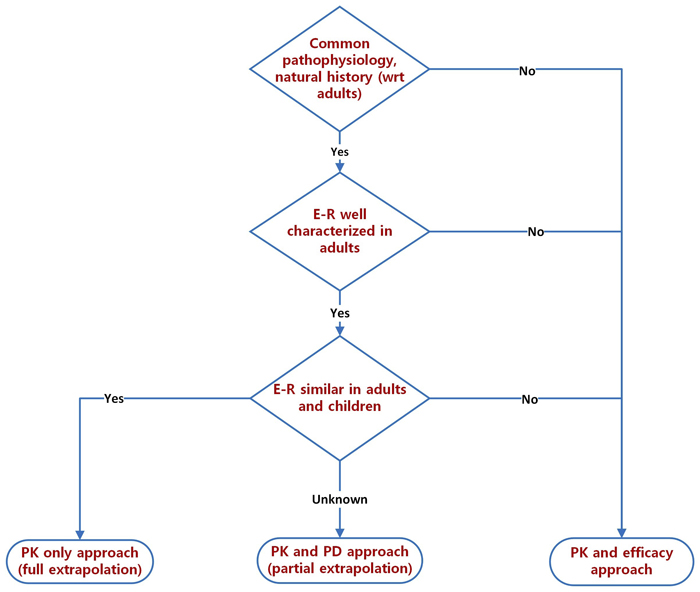
by admin | September 2021 | Uncategorized
Optimizing the use of the extrapolation concept in pediatric investigational programs: Here are 4 key considerations. Drug development in pediatrics continues to be a substrate for the application of innovative tools and techniques. When a new drug is approved in the...

by admin | September 2021 | Uncategorized
Risk assessment is a continuous process beginning right from discovery through the life of the drug. While we collaborate closely with our discovery, non-clinical and clinical pharmacology colleagues in understanding safety issues, we, as physicians, constantly bring...

by admin | September 2021 | Uncategorized
Precedent often trumps science when it comes to drug development. A common refrain is, “This is what we did at xxx”, “This is how they did it” and so on. So much so that we are doing things because that was how it was done. While there are many reasons to not...