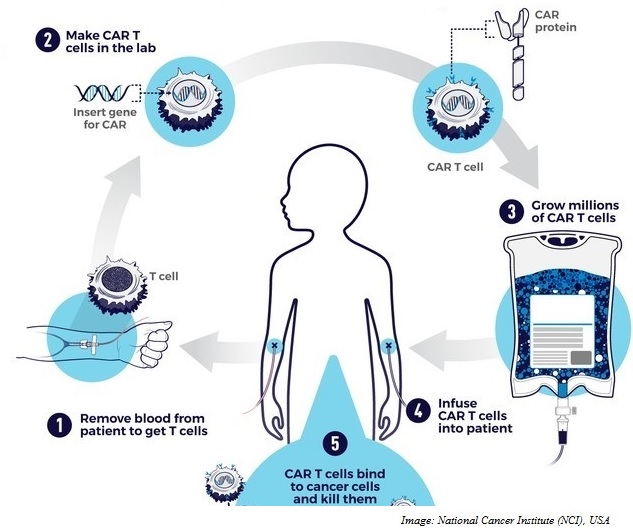
What should one look for when prioritizing cell therapy assets?
The phenomenal success of CARs in hematological malignancies has been driving the push towards development of CARs against solid tumors. We are often asked to help a biotech prioritize its CAR T assets.
In this blog, we review ways to prioritize a pipeline of hypothetical CAR T products for commercialization. We make our recommendations based on mechanism of action, competitive landscape and regulatory pathway. Being scientists, here, we focus on the scientific aspects of prioritization.
From the perspective of mechanism of action, we look at the target antigen, the CAR T product and their interaction.
There are several target antigen variables that are critical for prioritization. These include target antigen affinity (KD50) and target effects (EC50). The lower the values, the more effective the CAR. The antigen location is critical, CARs being the preferred modality for cell surface antigens and TCRs for intracellular antigens. Antigens could be tumor specific (e.g. EGFRvIII in malignant gliomas), tissue-specific (e.g. PSMA in prostate cancer) or could occur as part of normal development (CEA in adenocarcinomas) in descending order of specificity. A more specific antigen is always preferable for targeting. The role of the antigen in tumor pathogenesis, whether causal (e.g. PSMA in prostate cancer) or consequential (e.g MUC1 in pancreatic cancer) is useful information.
Typically, consequential antigens have better purchase as a target, given that they mark tumor tissues. The distribution of the antigen (whether primary or metastasis or both) determines the potential utility of the CAR. Antigens distributed in both the primary and metastatic lesions (e.g. GFRα4) make for ideal targets. Some of the other antigen parameters evaluated are proportion of patients with antigen, proportion of tumor cells expressing antigen, antigen load per tumor cell, and antigenicity.
An important aspect of target antigen is off-tumor expression. Whether the antigen is present in essential cells (e.g. NYESO-1 expressed in testis, embryonic ovaries, placenta), replaceable/non-essential cells (e.g. MUC1, an aberrant protein typically not expressed outside tumor tissue) and the degree of expression in these cells determine the potential for off-target effects and safety of the CAR. The effector–target (ET) ratio is another important variable as it is a determinant of CAR cell expansion and cytolytic activity. CAR therapies that allow for high E:T ratios have greater cytolytic activity compared to therapies that can achieve low E:T ratios.
Other CAR/TCR-specific efficacy variables considered are CAR modifications such as signaling domains (co-stimulatory domains like CD28 or 4-1BB) that add to the cytotoxic efficiency of the CAR, presence of switch receptors (small molecule switches to activate or inactivate CARs) allowing for better regulation of CAR activity, and dominant negative receptors (protein constructs that lack domains for downstream signaling) that can boost CAR efficacy.
Important tumor-related variables evaluated are accessibility through systemic vasculature, since more accessible the tumor (e.g. pancreatic tumors), easier it is to target, CAR trafficking that can be affected by chemokines (breast tumors with relatively lesser inhibitory chemokines allow easy homing in), microenvironment barriers either physical (e.g. breast tumors with abundant stroma) or cellular barriers (e.g. medullary thyroid cancers that have immunologically active microenvironments) that impact cytotoxic activity.
For competitor CAR/TCRs, we look at their ET ratio, CAR generation, CAR enhancements like suicide genes (e.g. inducible Caspase9) to enhance CAR safety and regimen (requirement for lymphodepletion) and immunogenicity.
For more blogs, please visit: https://www.rxmd.com/insights